Our research operates at the crucial intersection of physics, chemistry, and biology, where these fields are mutually dependent and integrated into interdisciplinary studies. Physics provides the foundational principles for techniques like Raman spectroscopy and photothermal therapy (PTT), while chemistry plays a central role in designing nanomaterials such as plasmonic and lipid-based nanoparticles. Biology, in turn, allows us to apply these innovations to complex systems, such as using 3D tumor models to explore cancer therapies. This interconnected approach enables us to create more comprehensive solutions, where advances in one area directly inform and enhance discoveries in the others, driving our goal of translating research into clinical applications.
- Nanomedicine
- Biomedicine
- Machine learning
- Raman Spectroscopy

Our lab focuses on the development and application of lipid-based and inorganic nanoparticles. These nanoparticles are engineered for targeted drug delivery, enhancing therapeutic efficacy while minimizing side effects. We are particularly interested in their potential for cancer treatment, where they can carry therapeutic agents directly to tumor cells, improving outcomes in chemo- and immunotherapy.

We utilize both 2D and 3D tumor models to simulate the tumor microenvironment for studying cancer progression and therapy. The 3D models, including spheroids and organoids, offer a more realistic representation of in vivo conditions, enabling us to better understand tumor dynamics and response to treatments in a controlled environment, especially in the context of brain and breast cancer.
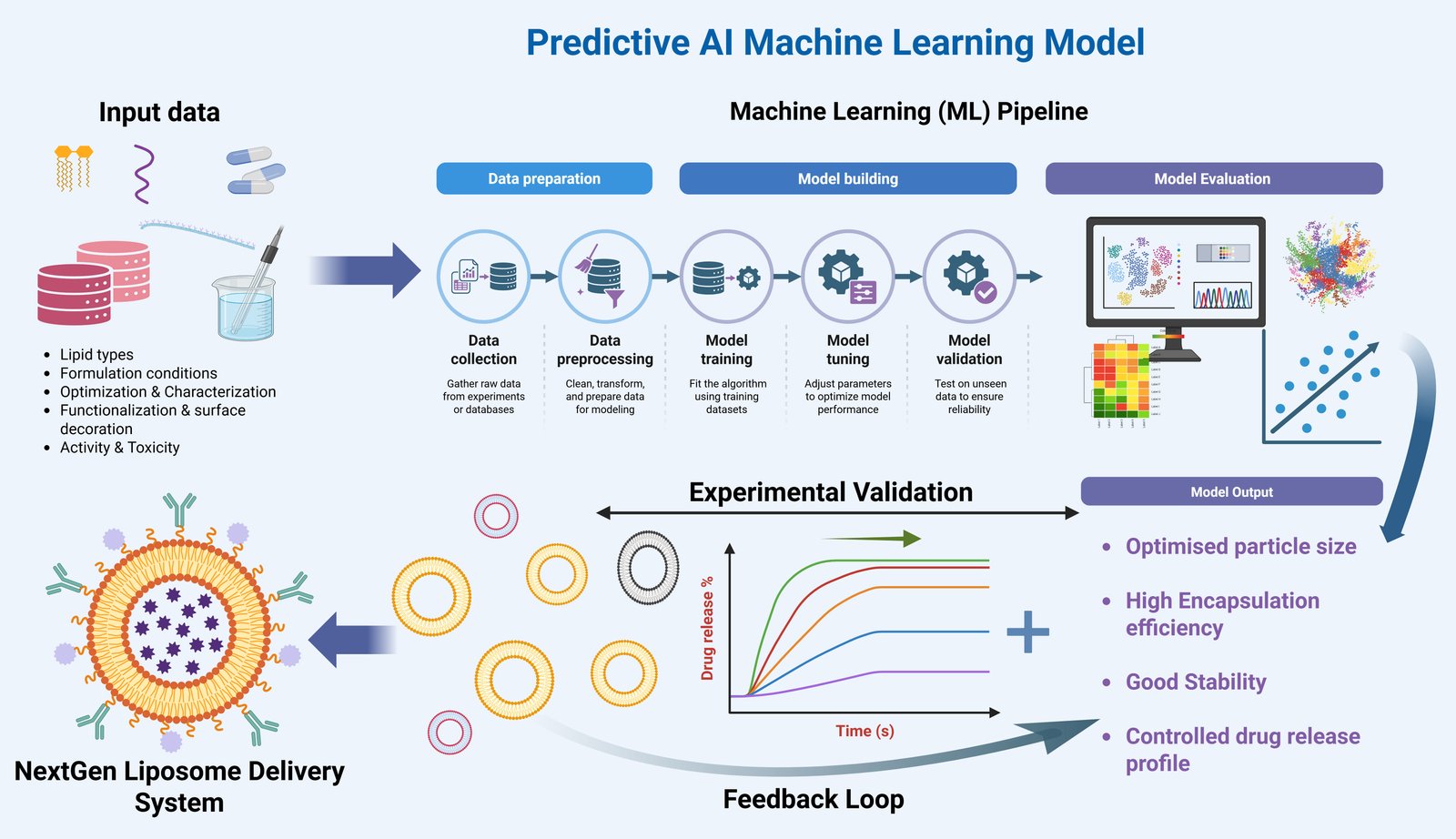
Our lab integrates AI and machine learning techniques to develop models for image processing, enabling precise analysis of complex biological data. Additionally, we use molecular dynamics simulations to model nano-bio interactions, providing insights into the behavior of nanoparticles within biological systems, which aids in optimizing nanomaterials for medical applications.

Our lab employs Raman spectroscopy to study the molecular fingerprint of biological samples, offering detailed insights into their biochemical composition. This non-destructive technique provides high specificity and sensitivity, making it ideal for detecting subtle changes in cellular structures. We focus on understanding how these molecular signatures shift during drug interactions, which helps in assessing treatment efficacy and cellular response. Additionally, we explore Surface-Enhanced Raman Spectroscopy (SERS) using plasmonic nanoparticles, which significantly amplifies Raman signals, allowing for the detection of low-abundance biomolecules and improving the precision of our analyses.